|
Introduction
A number of Livebearer Cyprinodontiformes are
euryhaline species, meaning that they can tolerate or withstand in a wide
range of water salinities.
Some species include populations living
simultaneously in fresh, brackish, or salt water habitats, comprising all
values of the salinity spectrum.
A quantity of other species can even be found
primarily in all sorts of protected and semi-protected marine and estuarine
habitats, like coastal marshes, mangrove swamps, estuaries, back waters and
in brackish water coastal lagoons.
A good number of the Poeciliidae and
Anablepidae members exist in quite saline water habitats and can even be
found in open ocean environments, at least for short periods, but most often
on the neighbouring coastal areas.
A very reduced number of species can even
endure in hyper saline ( brine ) niches, namely in desert lakes or ponds.
Although sometimes found in entirely fresh
water environments, Belonesox belizanus and a quantity of Jenynsia, Anableps
speceis, and several Micropecilia, Gambusia and Poecilia ones, among other
less known in the aquarium hobby, are usually found in coastal lagoons,
sandy or muddy bottom estuaries mangrove swamps as well as in all potential
brackish waters coastal habitats in the tropics and subtropics.
Relatively few species live primarily in clear
fast running watercourses, with rocky or large gravel bottoms and pure
crystalline fresh water and, as a rule; these are more commonly found among
Goodeids.
According to the salt-tolerant classification
of fresh-water fishes by George S. Myers ( 1949 ), many of the Livebearer
Cyprinodontiformes can be classified as “ secondary “, meaning “ rather
strictly confined to fresh water but relatively salt-tolerant, at least for
short periods “ and “ sporadic “ or “ fishes which live and breed
indifferently in salt or fresh water “.
Salinity
For the specific purpose of this website, we can define salinity, as the
concentration of dissolved salts in water.
As so many species of Livebearer Cyprinodontiformes prefer or require
brackish water habitats to stay alive and well, or in order to be kept in
hale and hearty conditions, it is advisable to have some notions about this
subject and how to control it in the aquarium hobby.
The measurement of salinity in professional circles is usually expressed in
“ parts per thousand “ ( ppt ). In the fish keeping hobby, in contrast, the
concentration of dissolved salts in water commonly uses the specific gravity
( sg ) notion.
As matter of fact, objects tend to float higher as major is the water
salinity, because in saline concentrations, like on sea, the water is
heavier per unit of volume ( denser ).
Thanks to this concept it becomes quite easily and quickly to measure
salinity through the use of a hydrometer, particularly with those
specifically prepared for the hobby, which are reasonably priced and easily
obtained.
Later on, some notions about how to use a hydrometer will be explained in
plain words.
Conventional classification system of water bodies based upon salinity on
the fish keeping hobby
|
Fresh water |
Brackish water |
Saline water |
Hyper saline water |
|
less than 0.05 % |
from 0.05% up to 3 % |
from 3% up to 5 % |
more than 5 % |
|
less than 0.5 ppt |
from 0.5 up to 30 ppt |
from 30 up to 50 ppt |
more than 50 ppt |
Fresh water is generally found in continents and islands. It results from
precipitation ( rain and snow fall ) and it flows naturally to the ocean, if
not retained.
On the transition places were fresh water is mixed with marine water, we can
locate brackish water. But this kind of water can also be found in
continental regions, incredibly far away from estuaries and coastal areas.
This is the case of some basins or lakes where the magnitude of
precipitation is lower than the evaporation level; consequently, when the
water evaporates the salts don’t and when this happens… salinity increases.
Besides the above mentioned continental locations, brackish, but primarily
saline waters are characteristically marine waters and are concentrated in
every ocean around the planet.
Another term for ocean or marine water is Euhaline Sea.
The widespread salinity of seas is generally comprised between 30 and 35 ppt,
depending on the ocean spot we are talking about.
Metahaline seas can contain from 36 up to 40 ppt, relating to the
concentration of dissolved salts in water.
On the topmost 35 meters of the Dead Sea, in Middle East, the salinity can
ranges between 300 and 400 ppt. It’s unquestionably an extreme paradigm of a
Brine Sea.
The technical term for salinity in marine environments ( oceans ) is
halinity. This is due to the fact that halides - chloride specifically - are
the most abundant anions in the mix of dissolved elements of sea water.
Total Molal Composition of Seawater ( salinity = 35 parts per
thousand )
|
Component |
Concentration ( mol / kg ) |
|
H2O ( Water ) |
53.6 |
|
Cl- ( Chloride ) |
0.546 |
|
Na+ ( Sodium ) |
0.469 |
|
Mg2+ ( Magnesium ) |
0.0528 |
|
SO42- ( Sulfate ) |
0.0282 |
|
Ca2+ ( Calcium ) |
0.0103 |
|
K+ ( Potassium ) |
0.0102 |
|
CT ( Total Inorganic Carbon ) |
0.00206 |
|
Br- ( Bromide ) |
0.000844 |
|
BT ( Total Boron ) |
0.000416 |
|
Sr2+ ( Strontium ) |
0.000091 |
|
F- ( Fluoride ) |
0.000068 |
|
|
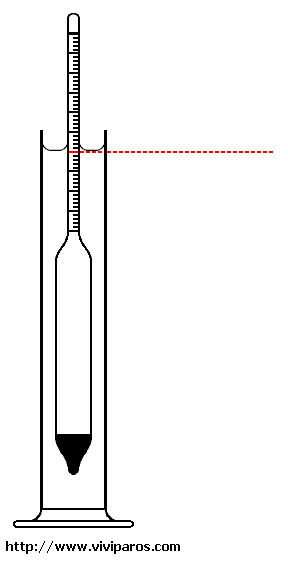
Figure 1 |
How to use a standard (
universal )
hydrometer to evaluate salinity
Monitoring the water salinity in your tank when you keep fish in brackish or
salt water is a must.
To determine water salinity on a professional way, the hobbits needs to make
use of equipments which are generally quite expensive.
Therefore, there is a less pricey and more practical approach to determine
the water salt concentration in your aquarium, which is by estimating
specific gravity with the use of a hydrometer.
A hydrometer is an instrument used to evaluate the specific gravity ( or
relative density ) of water and other liquids.
Usually it compares the ratio of the density of one liquid to the density of
plain fresh water, but it also works very well for contrast salt ( denser )
water with fresh water.
A standard hydrometer is usually made of glass, but today in the hobby we
can find other less precise models made of plastic that have a different way of working.
The traditional type consists of a cylindrical stem and a bulb, weighted with
mercury on the base to make it float upright.
The test water is poured into a tall container ( hydrometer jar ), usually in form of a long
glass or transparent plastic cylinder supported by a base.
The hydrometer is gently lowered into the water on the jar until it floats
freely ( see procedure n.º 3 downwards ).
The reading uses as reference the point at which the surface of the water
matches the stem of the hydrometer. Inside the stem, there is a paper scale,
so the specific gravity can be read directly.
Specific gravity can be defined as the ratio of water densities at various
temperatures, depending on the concentration of dissolved salts on it.
Since there is a direct relation to water temperature, if your hydrometer
doesn’t compensate automatically that factor, it should be regulated to the
temperature in your tank.
As a rule hydrometers are predominantly calibrated at 15ºC ( 59°F ), so the
reading outcome from a standard type still need to be converted to get the
actual or true specific gravity of the water.
On the other hand, changes in temperature do affect the densities of
materials in general, and the relative densities readings must be corrected
at certain temperatures, using a table.
For this purpose you can check your readings with the table of the measured
densities of water at various temperatures as the one on the link down below.
But to work with a standard ( universal ) model there are a few simple instructions :
1. Make sure that the instrument ( hydrometer ) is absolutely clean and
especially free from previous salt deposits. Wash it with purified
freshwater to remove every deposits. If some vestige will not be easily
dissolved with fresh water, try washing it with some acid, such as vinegar.
A cautious cleanse after every use, will prevent future clean-up
complicatedness.
2. Add enough water into the monitoring container so that the hydrometer can
float freely, particularly without touching the sides or bottom of it.
|
|
3. Lower your hydrometer smoothly, holding it
by the very pinnacle of the stem. Release the instrument into the water
while simultaneously forcing it a to tiny spin ( in order to displace any
bubbles fixed at the hydrometer ).
4. Ensure yourself that there are no visible air bubbles attach to the
instrument. Even tiny bubbles will facilitate floating and give you a
falsely reading. Pour slowly, and then stir gently.
5. The part of the hydrometer above the water level must be dry and spotless.
Sinking deeply, while place the hydrometer in water, it will leave water
remnants on the exposed part. Such extra weigh will drag down the hydrometer
and give a fallaciously low specific gravity reading.
6. Make sure that the hydrometer temperature is just about the same as the
water’s one.
7. Take a read from the hydrometer at the plane of the water's surface, not
along the edge of water where the liquid touches the glass, as the liquid
may curve to meet the sides and rises up along the stem of the hydrometer or
along the wall of the container ( see red line in figure 1 ).
8. Never leave a hydrometer floating around between uses.
Deposits may form this way and that will be
difficult to remove later.
A cautious clean after every use, will prevent
a tough concentrated effort after.
If necessary to add some saline solution on your tank’s water, use another
container to prepare the blend. Never perform salt mixes straightforward in
the aquarium water; unless there are no fish, invertebrates or plants
present or in case of emergency. In any circumstances act always very
carefully and be gentle.
Using the above mentioned additional container, add salt slowly until the
specific gravity reading reaches half of the required limit. Stir the water
for a few minutes to facilitate salt assimilation.
Remove and clean the hydrometer, and let the salt water latent for 36 hours.
After this period take another reading.
Continue to add salt until your hydrometer read reaches at the appropriate
level for the fish requirements at the environment that will receive the new
water. Stir the water for a few minutes to facilitate salt assimilation.
Remove and clean the hydrometer, and let the salt water latent for 24 hours.
After this period take another reading and if the level is proper uses the
new water without restraint, otherwise makes the final saline calibration
before transfer the water in to the destiny.
Handheld Refractometer
Another option for the concentration of dissolved salts in water in the
aquarium hobby is the handheld refractometer. It’s no doubt more expensive
than a standard hydrometer, but is becoming more and more popular and soon
it will be more low-priced for sure.
The handheld refractometer measures the concentrations of substances in
aqueous solutions using refracted light.
The process is a lot easier and more precise than with the standard
hydrometer.
You basically place the prism into your tank and watch as an angle is formed
on a scale, which indicates precisely the amount of dissolved solids.
After every use just wash the prism and you are ready to take your next
reading.
Most basic kits include prism assembly, daylight plate, rubber grip,
eyepiece with focus adjustment, and handy case.
The handheld refractometer performs automatic temperature compensation,
within a 20 degree range.
A few last words about density measurement
The International System of Units ( SI ) it is the world's most widely used
system of measurement.
The SI unit for density is kilograms per cubic meter ( kg/m³ ).
At 3.8° C ( 39.16º F ), water has a density of around 1000 kg/m³, making
this a convenient unit, but kilograms per cubic decimetre ( kg/dm³ ), grams
per millilitre ( g/mL ), or grams per cubic centimetre ( g/cc or g/cm³ ) are
all numerically equivalent ( 1 kg/L = 1 kg/dm³ = 1 g/cm³ = 1 g/mL ).
Determining the specific gravity ( the same as relative density of a
material relative to water ) requires little effort and is a simple, as the
density only needs to be divided by 1 or 1000, depending on the unit.
|
Temp ( ºC ) |
Temp ( ºF ) |
Density |
Correction relative to 15ºC ( 59º F ) |
|
0 |
32.00 |
0.99987 |
0.74 |
|
3.98 |
39.16 |
1.00000 |
0.87 |
|
5 |
41.00 |
0.99999 |
0.86 |
|
10 |
50.00 |
0.99973 |
0.60 |
|
15 |
59.00 |
0.99913 |
0.00 |
|
18 |
64.40 |
0.99862 |
0.51 |
|
20 |
68.00 |
0.99823 |
0.90 |
|
25 |
77.00 |
0.99707 |
2.06 |
|
30 |
86.00 |
0.99567 |
3.46 |
|
40 |
104.00 |
0.99224 |
6.89 |
|
50 |
122.00 |
0.98807 |
11.06 |
|
60 |
140.00 |
0.98324 |
15.89 |
|
70 |
158.00 |
0.97781 |
21.32 |
|
80 |
176.00 |
0.97183 |
27.30 |
|
90 |
194.00 |
0.96534 |
33.79 |
To compensate or correct your readings regarding temperatres, please follow
the Conversion
of Hydrometric Readings at any Temperature to Density Table.
To compare your density reading with salinity
in ppt, please follow the
Corresponding
Densities and Salinities Conversion Table.
Mathematically, the formula for density calculation is - (d = m/v); where D
is the density, M is the mass and V is the volume.
Although we use the above mentioned metric system, there are other metric
units outside the International System of Units.
In U.S. customary units or Imperial Units, the units of density include :
ounces per cubic inch ( oz/cu in )
pounds per cubic inch ( lb/cu in )
pounds per cubic foot ( lb/cu ft )
pounds per cubic yard ( lb/cu yd )
pounds per gallon ( for U.S. or imperial gallons ) ( lb/gal )
pounds per U.S. bushel ( lb/bu ).
|